|
|
Structure
of the human k-opioid receptor in complex with JDTic Huixian
Wu, Daniel Wacker, Mauro Mileni, Vsevolod Katritch, Gye Won
Han, Eyal Vardy, Wei Liu, Aaron A. Thompson, Xi-Ping Huang,
F. Ivy Carroll, S. Wayne Mascarella, Richard B. Westkaemper,
Philip D. Mosier, Bryan L. Roth, Vadim Cherezov & Raymond
C. Stevens
Abstract
"Opioid receptors mediate the actions
of endogenous and exogenous opioids on many physiological
processes, including the regulation of pain, respiratory drive,
mood, and—in the case of k-opioid receptor (k-OR)—dysphoria
and psychotomimesis. Here we report the crystal structure
of the human k-OR in complex with the selective antagonist
JDTic, arranged in parallel dimers, at 2.9Å resolution. The
structure reveals important features of the ligand-binding
pocket that contribute to the high affinity and subtype selectivity
of JDTic for the human k-OR. Modelling of other important
k-OR-selective ligands, including the morphinan-derived antagonists
norbinaltorphimine and 5'-guanidinonaltrindole, and the diterpene
agonist salvinorin A analogue RB-64, reveals both common and
distinct features for binding these diverse chemotypes. Analysis
of site-directed mutagenesis and ligand structure–activity
relationships confirms the interactions observed in the crystal
structure, thereby providing a molecular explanation for k-OR
subtype selectivity, and essential insights for the design
of compounds with new pharmacological properties targeting
the human k-OR."
reference
|
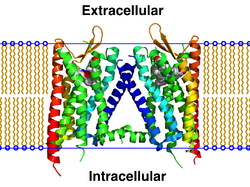 |
Crystallographic
structure of the human ?-opioid receptor homo dimer (4djh)
imbedded in a cartoon representation of a lipid bilayer. Each
monomer is individually rainbow color-ed (N-terminus = blue,
C-terminus = red). The receptor is bound to the ligand JDTic.[1]
Image
from Wikipedia.com
Note:
In this new image, the display of lysozyme is hidden.
|
ABOUT
THE K-OPIOID RECEPTOR
The
k-opioid receptor (KOR) is a protein that in humans
is encoded by the OPRK1 gene. The k-opioid receptor is one
of five related receptors that bind opium-like compounds in the
brain and are responsible for mediating the effects of these compounds.
These effects include altering the perception of pain, consciousness,
motor control, and mood.
The k-opioid
receptor is a type of opioid receptor that binds the opioid peptide
dynorphin as the primary endogenous ligand.[2]
In addition to dynorphin, a variety of natural alkaloids and synthetic
ligands bind to the receptor. The k-opioid receptor may provide
a natural addiction control mechanism, and consequently selective
agonists of this receptor may have therapeutic potential in the
treatment of addiction.
Distribution
k-Opioid receptors
are widely distributed in the brain (hypothalamus, periaqueductal
gray, and claustrum), spinal cord (substantia gelatinosa), and in
pain neurons.[3][4]
Subtypes
Based on receptor
binding studies, three variants of the k-opioid receptor designated
k1, k2, and k3 have been characterized.[5][6]
However only one cDNA clone has been identified,[7] hence these
receptor subtypes likely arise from interaction of one k-opioid
receptor protein with other membrane associated proteins.[8]
Function
It has long
been understood that k-opioid receptor agonists are dysphoric[9] but dysphoria
from k-opioid receptor agonists has been shown to differ between
the sexes.[10][11]
More recent studies have shown the aversive properties in a variety
of ways[12]
and the k-opioid receptor has been strongly implicated as an integral
neurochemical component of addiction and the remission thereof.
It is now widely
accepted that k-opioid receptor (partial) agonists have dissociative
and deliriant effects, as exemplified by salvinorin A. These effects
are generally undesirable in medicinal drugs and could have had
frightening or disturbing effects in the tested humans. It is thought
that the hallucinogenic effects of drugs such as butorphanol, nalbuphine,
and pentazocine serve to limit their opiate abuse potential. In
the case of salvinorin A, a structurally novel neoclerodane diterpene
κ-opioid receptor agonist, these hallucinogenic, more specifically
deliriant and dissociative, effects are sought after, even though
the experience is often considered dysphoric by the user. While
salvinorin A is considered a hallucinogen, it is not a psychedelic,
and its effects are qualitatively different than those produced
by the classical psychedelic hallucinogens such as LSD or mescaline.[13]
The involvement
of the k-opioid receptor in stress response has been elucidated.[9]
Activation of
the k-opioid receptor appears to antagonize many of the effects
of the k-opioid receptor.[14]
k-Opioid receptor
ligands are also known for their characteristic diuretic effects,
due to their negative regulation of antidiuretic hormone (ADH).[15]
k-Opioid agonism
is neuroprotective against hypoxia/ischemia; as such, k-opioid receptors
may represent a novel therapeutic target.[16]
Signal
transduction
k-Opioid receptor
activation by agonists is coupled to the G protein Gi/G0,
which subsequently increases phosphodiesterase activity. Phosphodiesterases
break down cAMP, producing an inhibitory effect in neurons.[17][18][19]
k-Opioid receptors also couple to inward-rectifier potassium[20]
and to N-type calcium ion channels.[21] Recent studies
have also demonstrated that agonist-induced stimulation of the κ-Opioid
receptor, like other G-protein coupled receptors, can result in
the activation of mitogen-activated protein kinases (MAPK). These
include extracellular signal-regulated kinase, p38 MAP kinases,
and c-Jun N-terminal kinases.[22][23][24][25][26][27]
Ligands
The synthetic
alkaloid ketazocine[28]
and terpenoid natural product salvinorin A[13]
are potent and selective k-opioid receptor agonists. The k-opioid
receptor also mediates the action of the hallucinogenic side effects
of opioids such as pentazocine.[29]
Agonists
:Asimadoline ; Bremazocine; Butorphanol; BRL-52537; Cyclazocine;
Dextromethorphan; Dynorphin (endogenous peptide ligand)
Antagonists:
5'-Guanidinonaltrindole; Buprenorphine ; Norbinaltorphimine; JDTic
Natural
agonists
Found in numerous
species of mint, (including peppermint, spearmint, and watermint),
the naturally-occurring compound Menthol is a weak k-opioid receptor
agonist[33]
owing to its antinociceptive effects in rats. In addition, mints
can desensitize a region through the activation of TRPM8 receptors
(the 'cold'/menthol receptor).[34]
Role
in treatment of drug addiction
k-Opioid agonists
have recently been investigated for their therapeutic potential
in the treatment of addiction[38]
and evidence points towards dynorphin, the endogenous k-opioid agonist,
to be the body's natural addiction control mechanism.[39]
Childhood stress/abuse is a well known predictor of drug abuse and
is reflected in alterations of the k- and k-opioid systems.[40]
In experimental "addiction" models the k-opioid receptor has also
been shown to influence stress-induced relapse to drug seeking behavior.
For the drug dependent individual, risk of relapse is a major obstacle
to becoming drug free. Recent reports demonstrated that k-opioid
receptors are required for stress-induced reinstatement of cocaine
seeking.[41][42]
One area of
the brain most strongly associated with addiction is the nucleus
accumbens (NAcc) and striatum while other structures that project
to and from the NAcc also play a critical role. Though many other
changes occur, addiction is often characterized by the reduction
of dopamine D2 receptors in the NAcc.[43] In addition
to low NAcc D2 binding,[44][45]
cocaine is also known to produce a variety of changes to the primate
brain such as increases prodynorphin mRNA in caudate putamen (striatum)
and decreases of the same in the hypothalamus while the administration
of a k-opioid agonist produced an opposite effect causing an increase
in D2 receptors in the NAcc.[46]
Additionally,
while cocaine overdose victims showed a large increase in k-opioid
receptors (doubled) in the NAcc,[47]
k-opioid agonist administration is shown to be effective in decreasing
cocaine seeking and self-administration.[48]
Furthermore, while cocaine abuse is associated with lowered prolactin
response,[49]
k-opioid activation causes a release in prolactin,[50]
a hormone known for its important role in learning, neuronal plasticity
and myelination.[51]
It has also
been reported that the k-opioid system is critical for stress-induced
drug-seeking. In animal models, stress has been demonstrated to
potentiate cocaine reward behavior in a kappa opioid-dependent manner.[52][53]
These effects are likely caused by stress-induced drug craving that
requires activation of the k-opioid system. Although seemingly paradoxical,
it is well known that drug taking results in a change from homeostasis
to allostasis. It has been suggested that withdrawal-induced dysphoria
or stress-induced dysphoria may act as a driving force by which
the individual seeks alleviation via drug taking[54]
The rewarding properties of drug are altered, and it is clear k-opioid
activation following stress modulates the valence of drug to increase
its rewarding properties and cause potentiation of reward behavior,
or reinstatement to drug seeking. The stress-induced activation
of k -opioid receptors is likely due to multiple signaling mechanisms.
The effects of k-opioid agonism on dopamine systems are well documented,
and recent work also implicates the mitogen-activated protein kinase
cascade and pCREB in k-opioid dependent behaviors. [25][55]
Though cocaine
abuse is a frequently used model of addiction, k-opioid agonists
have very marked effects on all types of addiction including alcohol
and opiate abuse.[12]
Not only are genetic differences in dynorphin receptor expression
a marker for alcohol dependence but a single dose of a k-opioid
antagonist markedly increased alcohol consumption in lab
animals.[56]
There are numerous studies that reflect a reduction in self-administration
of alcohol,[57]
and heroin dependence has also been shown to be effectively treated
with k-opioid agonism by reducing the immediate rewarding effects[58] and by causing
the curative effect of up-regulation of mu-opioid receptors[59]
that have been down-regulated during opioid abuse.
The anti-rewarding
properties of k-opioid agonists are mediated through both long-term
and short-term effects. The immediate effect of k-opioid agonism
leads to reduction of dopamine release in the NAcc during self administration
of cocaine[60]
and over the long term up-regulates receptors that have been down-regulated
during substance abuse such as mu-opioid and D2 receptors.
These receptors modulate the release of other neurochemicals such
as serotonin in the case of mu-opioid receptor agonists and acetylcholine
in the case of D2. These changes can account for the
physical and psychological remission of the pathology of addiction.
The longer effects of k-opioid agonism (30 minutes or greater) have
been linked to k-opioid receptor-dependent stress-induced potentiation
and reinstatement of drug seeking. It is hypothesized that these
behaviors are mediated by k-opioid-dependent modulation of dopamine,
serotonin, or norepinephrine and/or via activation of downstream
signal transduction pathways.
Selected Articles
Structure
of the human k-opioid receptor in complex with JDTic
Atomic
Structure of Molecule That Binds to Opioids in the Brain Discovered
RTI
International's JDTic Helps Scientists Uncover Structure of the
Kappa Opioid Receptor
References
- PDB
4DJH; Wu H, Wacker D, Mileni
M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP,
Carroll FI, Mascarella SW, Westkaemper RB, Mosier PD, Roth BL,
Cherezov V, Stevens RC (March 2012). "Structure of the human
κ-opioid receptor in complex with JDTic". Nature. doi:10.1038/nature10939.
PMID 22437504.
- James
IF, Chavkin C, Goldstein A (1982). "Selectivity of dynorphin
for kappa opioid receptors". Life Sci. 31 (12-13):
1331-4. doi:10.1016/0024-3205(82)90374-5. PMID 6128656.
-
Fine,
Perry G.; Russell K. Portenoy (2004). "Chapter 2: The Endogenous Opioid System". A Clinical
Guide to Opioid Analgesia. McGraw Hill.
http://www.stoppain.org/pcd/_pdf/OpioidChapter2.pdf.
- Mansour
A, Fox CA, Akil H, Watson SJ (January 1995). "Opioid-receptor mRNA expression in the rat CNS: anatomical and
functional implications". Trends Neurosci. 18
(1): 22-9. doi:10.1016/0166-2236(95)93946-U. PMID 7535487. http://linkinghub.elsevier.com/retrieve/pii/016622369593946U.
- de
Costa BR, Rothman RB, Bykov V, Jacobson AE, Rice KC (February
1989). "Selective and enantiospecific acylation of kappa opioid
receptors by (1S,2S)-trans-2-isothiocyanato-N-methyl-N-[2-(1-pyrrolidinyl)
cyclohexy l] benzeneacetamide. Demonstration of kappa receptor
heterogeneity". J. Med. Chem. 32 (2): 281-3. doi:10.1021/jm00122a001.
PMID 2536435.
- Rothman
RB, France CP, Bykov V, De Costa BR, Jacobson AE, Woods JH,
Rice KC (August 1989). "Pharmacological activities of optically
pure enantiomers of the kappa opioid agonist, U50,488, and its
cis diastereomer: evidence for three kappa receptor subtypes".
Eur. J. Pharmacol. 167 (3): 345-53. doi:10.1016/0014-2999(89)90443-3. PMID 2553442.
- Mansson
E, Bare L, Yang D (August 1994). "Isolation of a human kappa
opioid receptor cDNA from placenta". Biochem. Biophys. Res.
Commun. 202 (3): 1431-7. doi:10.1006/bbrc.1994.2091.
PMID 8060324.
-
Jordan
BA, Devi LA (June 1999). "G-protein-coupled receptor heterodimerization modulates receptor
function". Nature 399 (6737): 697-700. doi:10.1038/21441. PMC 3125690. PMID 10385123. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3125690.
- Land
BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C (January
2008). "The dysphoric component of stress is encoded by activation of
the dynorphin kappa-opioid system". J. Neurosci.
28 (2): 407-14. doi:10.1523/JNEUROSCI.4458-07.2008. PMC 2612708. PMID 18184783. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2612708.
- Lomas
LM, Barrett AC, Terner JM, Lysle DT, Picker MJ (April 2007).
"Sex differences in the potency of kappa opioids and mixed-action
opioids administered systemically and at the site of inflammation
against capsaicin-induced hyperalgesia in rats". Psychopharmacology
(Berl.) 191 (2): 273-85. doi:10.1007/s00213-006-0663-1. PMID 17225166.
-
Sershen
H, Hashim A, Lajtha A (August 1998). "Gender differences in
kappa-opioid modulation of cocaine-induced behavior and NMDA-evoked
dopamine release". Brain Res. 801 (1-2): 67-71.
doi:10.1016/S0006-8993(98)00546-0. PMID 9729284.
- Xuei
X, Dick D, Flury-Wetherill L, Tian HJ, Agrawal A, Bierut L,
Goate A, Bucholz K, Schuckit M, Nurnberger J, Tischfield J,
Kuperman S, Porjesz B, Begleiter H, Foroud T, Edenberg HJ (November
2006). "Association of the kappa-opioid system with alcohol
dependence". Mol. Psychiatry 11 (11): 1016–24.
doi:10.1038/sj.mp.4001882.
PMID 16924269.
- Roth
BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S,
Ernsberger P, Rothman RB (2002). "Salvinorin A: a potent naturally occurring nonnitrogenous kappa
opioid selective agonist". Proc. Natl. Acad. Sci. U.S.A.
99 (18): 11934-9. doi:10.1073/pnas.182234399.
PMC 129372. PMID 12192085. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=129372.
-
Pan
ZZ (1998). "mu-Opposing actions of the kappa-opioid receptor".
Trends Pharmacol. Sci. 19 (3): 94–8. doi:10.1016/S0165-6147(98)01169-9. PMID 9584625.
- Yamada
K, Imai M, Yoshida S (1989). "Mechanism of diuretic action of
U-62,066E, a kappa opioid receptor agonist". Eur. J. Pharmacol.
160 (2): 229-37. doi:10.1016/0014-2999(89)90495-0. PMID 2547626.
- Zeynalov
E, Nemoto M, Hurn PD, Koehler RC, Bhardwaj A (2006). "Neuroprotective
effect of selective kappa opioid receptor agonist is gender
specific and linked to reduced neuronal nitric oxide". J.
Cereb. Blood Flow Metab. 26 (3): 414–20. doi:10.1038/sj.jcbfm.9600196.
PMID 16049424.
- Lawrence
DM, Bidlack JM (September 1993). "The kappa opioid receptor expressed on the mouse R1.1 thymoma
cell line is coupled to adenylyl cyclase through a pertussis
toxin-sensitive guanine nucleotide-binding regulatory protein".
J. Pharmacol. Exp. Ther. 266 (3): 1678-83. PMID 8103800. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=8103800.
- Konkoy
CS, Childers SR (January 1993). "Relationship between kappa 1 opioid receptor binding and inhibition
of adenylyl cyclase in guinea pig brain membranes". Biochem.
Pharmacol. 45 (1): 207-16. doi:10.1016/0006-2952(93)90394-C. PMID 8381004. http://linkinghub.elsevier.com/retrieve/pii/0006-2952(93)90394-C.
- Schoffelmeer
AN, Rice KC, Jacobson AE et al (September 1988). "Mu-, delta- and kappa-opioid receptor-mediated inhibition of
neurotransmitter release and adenylate cyclase activity in rat
brain slices: studies with fentanyl isothiocyanate". Eur.
J. Pharmacol. 154 (2): 169-78. doi:10.1016/0014-2999(88)90094-5. PMID 2906610. http://linkinghub.elsevier.com/retrieve/pii/0014-2999(88)90094-5.
- Henry
DJ, Grandy DK, Lester HA, Davidson N, Chavkin C (March 1995).
"Kappa-opioid receptors couple to inwardly rectifying potassium
channels when coexpressed by Xenopus oocytes". Mol. Pharmacol.
47 (3): 551–7. PMID 7700253. http://molpharm.aspetjournals.org/cgi/pmidlookup?view=long&pmid=7700253.
- Tallent
M, Dichter MA, Bell GI, Reisine T (December 1994). "The cloned kappa opioid receptor couples to an N-type calcium
current in undifferentiated PC-12 cells". Neuroscience
63 (4): 1033-40. doi:10.1016/0306-4522(94)90570-3. PMID 7700508. http://linkinghub.elsevier.com/retrieve/pii/0306-4522(94)90570-3.
- Bohn
LM, Belcheva MM, Coscia CJ (February 2000). "Mitogenic signaling via endogenous kappa-opioid receptors in
C6 glioma cells: evidence for the involvement of protein kinase
C and the mitogen-activated protein kinase signaling cascade".
J Neurochem 74 (2): 564-73. doi:10.1046/j.1471-4159.2000.740564.x. PMC 2504523. PMID 10646507. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2504523.
-
Belcheva
MM, Clark AL, Haas PD, Serna JS, Hahn JW, Kiss A, Coscia CJ
(July 2005). "Mu and kappa opioid receptors activate ERK/MAPK via different
protein kinase C isoforms and secondary messengers in astrocytes".
J. Biol. Chem. 280 (30): 27662-9. doi:10.1074/jbc.M502593200.
PMC 1400585. PMID 15944153. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1400585.
- Bruchas
MR, Macey TA, Lowe JD, Chavkin C (June 2006). "Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent
in neurons and astrocytes". J. Biol. Chem. 281
(26): 18081-9. doi:10.1074/jbc.M513640200.
PMC 2096730. PMID 16648139. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2096730.
- Bruchas
MR, Xu M, Chavkin C (September 2008). "Repeated swim stress induces kappa opioid-mediated activation
of extracellular signal-regulated kinase 1/2". Neuroreport
19 (14): 1417-22. doi:10.1097/WNR.0b013e32830dd655. PMC 2641011. PMID 18766023. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2641011.
- Kam
AY, Chan AS, Wong YH (July 2004). "Kappa-opioid receptor signals
through Src and focal adhesion kinase to stimulate c-Jun N-terminal
kinases in transfected COS-7 cells and human monocytic THP-1
cells". J. Pharmacol. Exp. Ther. 310 (1): 301-10.
doi:10.1124/jpet.104.065078.
PMID 14996948.
- Bruchas
MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S, Chavkin C
(October 2007). "Long-acting kappa opioid antagonists disrupt receptor signaling
and produce noncompetitive effects by activating c-Jun N-terminal
kinase". J. Biol. Chem. 282 (41): 29803-11.
doi:10.1074/jbc.M705540200.
PMC 2096775. PMID 17702750. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2096775.
- Pasternak
GW (June 1980). "Multiple opiate receptors: [3Hethylketocyclazocine receptor
binding and ketocyclazocine analgesia"]. Proc. Natl. Acad.
Sci. U.S.A. 77 (6): 3691–4. doi:10.1073/pnas.77.6.3691.
PMC 349684. PMID 6251477. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=349684.
- Holtzman
SG (February 1985). "Drug discrimination studies". Drug Alcohol
Depend 14 (3-4): 263–82. doi:10.1016/0376-8716(85)90061-4. PMID 2859972.
- Wang
Y, Chen Y, Xu W, Lee DY, Ma Z, Rawls SM, Cowan A, Liu-Chen LY
(March 2008). "2-Methoxymethyl-salvinorin B is a potent kappa opioid receptor
agonist with longer lasting action in vivo than salvinorin A".
The Journal of Pharmacology and Experimental Therapeutics
324 (3): 1073-83. doi:10.1124/jpet.107.132142.
PMC 2519046. PMID 18089845. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2519046.
- Munro
TA, Duncan KK, Xu W, Wang Y, Liu-Chen LY, Carlezon WA, Cohen
BM, Béguin C (February 2008). "Standard protecting groups create potent and selective kappa
opioids: salvinorin B alkoxymethyl ethers". Bioorganic
& Medicinal Chemistry 16 (3): 1279-86. doi:10.1016/j.bmc.2007.10.067. PMC 2568987. PMID 17981041. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2568987.
- Baker
LE, Panos JJ, Killinger BA, Peet MM, Bell LM, Haliw LA, Walker
SL (April 2009). "Comparison of the discriminative stimulus
effects of salvinorin A and its derivatives to U69,593 and U50,488
in rats". Psychopharmacology 203 (2): 203-11.
doi:10.1007/s00213-008-1458-3. PMID 19153716.
- Galeotti
N, Di Cesare Mannelli L, Mazzanti G, Bartolini A, Ghelardini
C (April 2002). "Menthol: a natural analgesic compound". Neurosci.
Lett. 322 (3): 145-8. doi:10.1016/S0304-3940(01)02527-7. PMID 11897159.
- Werkheiser
JL, Rawls SM, Cowan A (October 2006). "Mu and kappa opioid receptor
agonists antagonize icilin-induced wet-dog shaking in rats".
Eur. J. Pharmacol. 547 (1-3): 101-5. doi:10.1016/j.ejphar.2006.07.026. PMID 16945367.
- Butelman
ER, Mandau M, Tidgewell K, Prisinzano TE, Yuferov V, Kreek MJ
(January 2007). "Effects of salvinorin A, a kappa-opioid hallucinogen,
on a neuroendocrine biomarker assay in nonhuman primates with
high kappa-receptor homology to humans". The Journal of pharmacology
and experimental therapeutics 320 (1): 300-6. doi:10.1124/jpet.106.112417.
PMID 17060493.
- Chavkin
C, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, Toth BA,
Hufeisen SJ, Roth BL (March 2004). "Salvinorin A, an active
component of the hallucinogenic sage salvia divinorum is a highly
efficacious kappa-opioid receptor agonist: structural and functional
considerations". The Journal of pharmacology and experimental
therapeutics 308 (3): 1197-203. doi:10.1124/jpet.103.059394.
PMID 14718611.
- Glick
SD, Maisonneuve IS (May 1998). "Mechanisms of antiaddictive
actions of ibogaine". Annals of the New York Academy of Sciences
844: 214-26. doi:10.1111/j.1749-6632.1998.tb08237.x. PMID 9668680.
- Hasebe
K, Kawai K, Suzuki T, Kawamura K, Tanaka T, Narita M, Nagase
H, Suzuki T (October 2004). "Possible pharmacotherapy of the
opioid kappa receptor agonist for drug dependence". Annals
of the New York Academy of Sciences 1025: 404-13.
doi:10.1196/annals.1316.050.
PMID 15542743.
- Frankel
PS, Alburges ME, Bush L, Hanson GR, Kish SJ (July 2008). "Striatal and ventral pallidum dynorphin concentrations are markedly
increased in human chronic cocaine users". Neuropharmacology
55 (1): 41-6. doi:10.1016/j.neuropharm.2008.04.019. PMC 2577569. PMID 18538358. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2577569.
-
Michaels
CC, Holtzman SG (April 2008). "Early postnatal stress alters
place conditioning to both mu- and kappa-opioid agonists". The
Journal of pharmacology and experimental therapeutics 325
(1): 313–8. doi:10.1124/jpet.107.129908.
PMID 18203949.
- Beardsley
PM, Howard JL, Shelton KL, Carroll FI (November 2005). "Differential
effects of the novel kappa opioid receptor antagonist, JDTic,
on reinstatement of cocaine-seeking induced by footshock stressors
vs cocaine primes and its antidepressant-like effects in rats".
Psychopharmacology (Berl.) 183 (1): 118-26. doi:10.1007/s00213-005-0167-4. PMID 16184376.
- Redila
VA, Chavkin C (September 2008). "Stress-induced reinstatement of cocaine seeking is mediated
by the kappa opioid system". Psychopharmacology (Berl.)
200 (1): 59-70. doi:10.1007/s00213-008-1122-y. PMC 2680147. PMID 18575850. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2680147.
- Blum
K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D,
Lubar JO, Chen TJ, Comings DE (November 2000). "Reward deficiency
syndrome: a biogenetic model for the diagnosis and treatment
of impulsive, addictive, and compulsive behaviors". Journal
of psychoactive drugs 32 Suppl: i–iv, 1–112.
PMID 11280926.
-
Stefański
R, Ziółkowska B, Kuśmider M, Mierzejewski P, Wyszogrodzka
E, Kołomańska P, Dziedzicka-Wasylewska M, Przewłocki R, Kostowski
W (July 2007). "Active versus passive cocaine administration:
differences in the neuroadaptive changes in the brain dopaminergic
system". Brain research 1157: 1–10. doi:10.1016/j.brainres.2007.04.074. PMID 17544385.
- Moore
RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP (September
1998). "Effect of cocaine self-administration on dopamine D2
receptors in rhesus monkeys". Synapse 30 (1):
88-96. doi:10.1002/(SICI)1098-2396(199809)30:1<88::AID-SYN11>3.0.CO;2-L.
PMID 9704885.
-
D'Addario
C, Di Benedetto M, Izenwasser S, Candeletti S, Romualdi P (January
2007). "Role of serotonin in the regulation of the dynorphinergic
system by a kappa-opioid agonist and cocaine treatment in rat
CNS". Neuroscience 144 (1): 157-64. doi:10.1016/j.neuroscience.2006.09.008. PMID 17055175.
- Mash
DC, Staley JK (June 1999). "D3 dopamine and kappa opioid receptor
alterations in human brain of cocaine-overdose victims". Annals
of the New York Academy of Sciences 877: 507-22.
doi:10.1111/j.1749-6632.1999.tb09286.x. PMID 10415668.
- Schenk
S, Partridge B, Shippenberg TS (June 1999). "U69593, a kappa-opioid
agonist, decreases cocaine self-administration and decreases
cocaine-produced drug-seeking". Psychopharmacology 144
(4): 339–46. doi:10.1007/s002130051016.
PMID 10435406.
- Patkar
AA, Mannelli P, Hill KP, Peindl K, Pae CU, Lee TH (August 2006).
"Relationship of prolactin response to meta-chlorophenylpiperazine
with severity of drug use in cocaine dependence". Human psychopharmacology
21 (6): 367-75. doi:10.1002/hup.780.
PMID 16915581.
- Butelman
ER, Kreek MJ (July 2001). "kappa-Opioid receptor agonist-induced
prolactin release in primates is blocked by dopamine D(2)-like
receptor agonists". European journal of pharmacology
423 (2-3): 243–9. doi:10.1016/S0014-2999(01)01121-9. PMID 11448491.
- Gregg
C, Shikar V, Larsen P, Mak G, Chojnacki A, Yong VW, Weiss S
(February 2007). "White matter plasticity and enhanced remyelination
in the maternal CNS". Journal of Neuroscience 27
(8): 1812-23. doi:10.1523/JNEUROSCI.4441-06.2007. PMID 17314279.
- McLaughlin
JP, Marton-Popovici M, Chavkin C. (July 2003). "Kappa opioid receptor antagonism and prodynorphin gene disruption
block stress-induced behavioral responses". Journal of
Neuroscience 23 (13): 5674–83. PMC 2104777. PMID 12843270. http://www.jneurosci.org/cgi/reprint/23/13/5674.
- Mash,
DEBORAH C.; Li, S; Valdez, J; Chavkin, TA; Chavkin, C (June
2006). "Social defeat stress-induced behavioral responses are mediated
by the endogenous kappa opioid system". Neuropsychopharmacology
31 (4): 787–94. doi:10.1038/sj.npp.1300872.
PMC 2096774. PMID 16123746. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2096774.
-
Koob
GF (July 2008). "A role for brain stress systems in addiction". Neuron
59 (1): 11-34. doi:10.1016/j.neuron.2008.06.012. PMC 2748830. PMID 18614026. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2748830.
- Bruchas
M. R., Land B. B., Aita M., Xu M., Barot S. K., Li S., Chavkin
C. (2007). "Stress-induced p38 mitogen-activated protein kinase activation
mediates -opioid-dependent dysphoria". J Neurosci
27 (43): 11614–23. doi:10.1523/JNEUROSCI.3769-07.2007. PMC 2481272. PMID 17959804. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2481272.
- Mitchell
JM, Liang MT, Fields HL (November 2005). "A single injection
of the kappa opioid antagonist norbinaltorphimine increases
ethanol consumption in rats". Psychopharmacology 182
(3): 384-92. doi:10.1007/s00213-005-0067-7. PMID 16001119.
- Walker
BM, Koob GF (February 2008). "Pharmacological evidence for a motivational role of kappa-opioid
systems in ethanol dependence". Neuropsychopharmacology
33 (3): 643-52. doi:10.1038/sj.npp.1301438.
PMC 2739278. PMID 17473837. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2739278.
- Xi
ZX, Fuller SA, Stein EA (January 1998). "Dopamine release in the nucleus accumbens during heroin self-administration
is modulated by kappa opioid receptors: an in vivo fast-cyclic
voltammetry study". The Journal of pharmacology and experimental
therapeutics 284 (1): 151-61. PMID 9435173. http://jpet.aspetjournals.org/cgi/content/abstract/284/1/151.
-
Narita
M, Khotib J, Suzuki M, Ozaki S, Yajima Y, Suzuki T (June 2003).
"Heterologous mu-opioid receptor adaptation by repeated stimulation
of kappa-opioid receptor: up-regulation of G-protein activation
and antinociception". Journal of neurochemistry 85
(5): 1171-9. doi:10.1046/j.1471-4159.2003.01754.x. PMID 12753076.
- Maisonneuve
IM, Archer S, Glick SD (November 1994). "U50,488, a kappa opioid
receptor agonist, attenuates cocaine-induced increases in extracellular
dopamine in the nucleus accumbens of rats". Neuroscience
letters 181 (1–2): 57-60. doi:10.1016/0304-3940(94)90559-2. PMID 7898771.
- Huang,
Peng; Steplock Deborah, Weinman Edward J, Hall Randy A, Ding
Zhe, Li Jianguo, Wang Yulin, Liu-Chen Lee-Yuan (Jun. 2004).
"kappa Opioid receptor interacts with Na(+)/H(+)-exchanger regulatory
factor-1/Ezrin-radixin-moesin-binding phosphoprotein-50 (NHERF-1/EBP50)
to stimulate Na(+)/H(+) exchange independent of G(i)/G(o) proteins".
J. Biol. Chem. (United States) 279 (24): 25002-9.
doi:10.1074/jbc.M313366200.
ISSN 0021-9258.
PMID 15070904.
- Li,
Jian-Guo; Chen Chongguang, Liu-Chen Lee-Yuan (Jul. 2002).
"Ezrin-radixin-moesin-binding phosphoprotein-50/Na+/H+ exchanger
regulatory factor (EBP50/NHERF) blocks U50,488H-induced down-regulation
of the human kappa opioid receptor by enhancing its recycling
rate". J. Biol. Chem. (United States) 277 (30):
27545–52. doi:10.1074/jbc.M200058200.
ISSN 0021-9258.
PMID 12004055.
-
Li,
Jian-Guo; Haines Dale S, Liu-Chen Lee-Yuan (Apr. 2008).
"Agonist-promoted Lys63-linked polyubiquitination of the human
kappa-opioid receptor is involved in receptor down-regulation".
Mol. Pharmacol. (United States) 73 (4): 1319-30.
doi:10.1124/mol.107.042846.
PMID 18212250.
|

