How do drugs work?Drugs generally work by interacting with receptors on the surface of cells or enzymes (which regulate the rate of chemical reactions) within cells. Receptor and enzyme molecules have a specific three-dimensional structure which allows only substances that fit precisely to attach to it. This is often referred to as a lock and key model. Most drugs work because by binding to the target receptor site, they can either block the physiological function of the protein, or mimics it's effect. If a drug causes the protein receptor to respond in the same way as the naturally occurring substance, then the drug is referred to as an agonist. Examples of agonists are morphine, nicotine, phenylephrine, and isoproterenol. Antagonists are drugs that interact selectively with receptors but do not lead to an observed effect. Instead they reduce the action of an agonist at the receptor site involved. Receptor antagonists can be classified as reversible or irreversible. Reversible antagonists readily dissociate from their receptor. Irreversible antagonists form a stable chemical bond with their receptor (eg, in alkylation). Examples of antagonist drugs are: beta-blockers, such as propranolol. Instead of receptors, some drugs target enzymes, which regulate the rate of chemical reactions. Drugs that target enzymes are classified as inhibitors or activators (inducers). Examples of drugs that target enzymes are: aspirin, cox-2 inhibitors and hiv protease inhibitors (see below). Many drug companies will design structural variants for compounds that bind receptor sites in hope of making a compound that is more effective. Until recently design of new drugs was very difficult. Scientists had no way to know what the binding site of the protein looked like. Scientist now have a powerful new tool. Molecular modeling allows researchers to view the 3-D structure of proteins and their binding sites using data from X-ray crystallography and NMR spectroscopy . The synthesis of several recent drugs (including HIV Protease Inhibitors for treatment of AIDS) have been assisted using the 3-D structure of protein. CASE I: HOW ASPIRIN AND OTHER NONSTEROIDAL ANTI-INFLAMMATORY INHIBITORS WORKS Nonsteroidal anti-inflammatory drugs work by interfering with the cyclooxygenase pathway. The normal process begins with arachidonic acid, a dietary unsaturated fatty acid obtained from animal fats. This acid is converted by the enzyme cyclooxygenase to synthesize different prostaglandins. The prostaglandins go on to stimulate many other regulatory functions and reactionary responses in the body including: inflammation and increased sensitivity to pain . Aspirin and other NSAID's work by inhibiting this pathway. Recent research has shown that there are two types of cyclooxygenase, denoted COX-1 and COX-2. Each type of cyclooxygenase lends itself to producing different types of prostaglandins. COX-1 is located in the stomach wall.
-------------->spin on -------->- spin off
------>space fill/cpk -------->stick ----> ball-and-stick pdb fle: 1CVU (shown using Jsmol)
By selectively binding the arachidonic acid site NSAID inhibit the COX-2 enzyme. Shown to left: Cyclooxygenase-2 complexed with a non-selective inhibitor, indomethacin (only the A chain is shown with heme and inhibitor molecule To view Backbone model with hetero atoms (heme and indomethacin). Right click --> Select --> Protein --> All Right Click -->Style --> Structure --> Backbone to Color Backbone by Amino Acid Color -->Structures --> Backbone--> Scheme --> Amino Acids 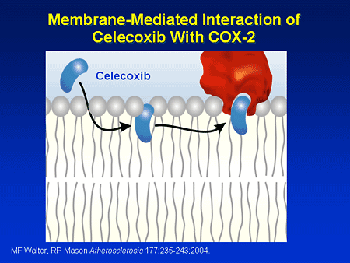
How do NSAID get to the active site?
Access to the COX-2 catalytic site is through the membrane lipid. Celecoxib intercalates into the membrane core and then diffuses along a path to gain access to the hydrophobid binding site Other drugs may use a slightly different mechanism providing evidence for the flexible nature of cyclooxygenase. What causes side effects in the case of aspirin? Why does aspirin cause cause stomach upset but the newer NSAID drugs do not? The two forms of cyclooxygenase have equal molecular weights and are very similar in structure. However, the binding active site of COX-1 (located in the stomach walls) is smaller than the similar site of COX-2, so it accepts a smaller range of structures as substrates. In the stomach COX-1 makes prostaglandin that seems to keep your stomach lining nice and thick by stimulating mucous production; inhibiting this enzyme can cause irritation of the stomach lining. CASE II: HOW DO AIDS ANTI-VIRAL DRUGS WORK? Protease inhibitors inhibit the activity of protease, an enzyme used by HIV to cleave nascent proteins for final assembly of new HIV virons, and so prevent viral replication. This was the second class of antiretroviral drugs developed. Indinavir -- Trade name: Crixivan® was FDA approved March 13, 1996. It was the eighth approved antiretroviral drug. Indinavir was much more powerful than any prior antiretroviral drug; using it with dual NRTIs set the standard for treatment of HIV/AIDS and raised the bar the design and introduction of subsequent antiretroviral drugs. Protease inhibitors changed the very nature of the AIDS epidemic from one of a terminal illness to a somewhat manageable one. -------------->spin on -------->- spin off ------>space fill/cpk -------->stick ----> ball-and-stick CRIXIVAN IS SHOWN BOUND TO THE PROTEASE ENZYME ACTIVE SITE. THE PROTEIN BACKBONE IS SHOWN COLORED BY AMINO ACID.
PDB FILE: 1HPV (shown using Jsmolt)
CASE IIII: HUMAN GROWTH HORMONE RECEPTOR Human growth hormone (pink) binds two receptor molecules (gold) and thereby induces signal transduction through receptor dimerization. Growth hormone is naturally produced by the pituitary gland and is necessary to stimulate growth in children. .
CASE IV: G -PROTEIN RECEPTOR G proteins are molecular switches that use GDP to control their signaling cycle. The G protein system plays a central role in many signaling tasks, making it a sensitive target for drugs and toxins. Many of the drugs that are currently on the market, such as Claritin and Prozac, as well as a number of drugs of abuse, such as heroin, cocaine and marijuana, act at G-protein-coupled receptors in these signaling chains. GPCRs are also involved in aging, cancer, cell growth stimulation, controlling metabolism .... For more information see G Proteins. -------------->spin on -------->- spin off ------>space fill/cpk -------->stick ----> ball-and-stick G protein with bound GDP molecule.
|
||||
Explain it with Molecules
- Why is water such a good solvent?
- Why does ice float?
- Why do solids, liquids and gases behave differently?
- What is the geometry of methane?
- What's the difference between alpha and beta glucose?
- How does caffeine work in the brain?
- How does soap work?
- What is the difference between sucrose and fructose?
- Why is carbon monoxide so dangerous?
- Why is graphite so soft if it is made of only carbon?
- What is the difference between Carbyne and Graphite?
- Why is the fullerene and similar structures the cornerstone of nanotechnology?
- How big is a nanotube?
- Why does table salt have a cubic crystal shape?
- What is the structure of the benzene molecule?
- Why do carcinogens cause cancer?
- What causes Sickle Cell Anemia?
- What is the difference between sodium nitrite and nitrate?
- How do drugs work?
World of Molecules
- The Periodic Table of Elements
- What is a Molecule?-- When is a Molecule also a Compound?
- 3D Structures using Jsmol/Jmol
- Explain it with Molecules
- Healthy Molecules from Exercise
- Acid and Base Molecules