|
What is the structure of the Carbyne molecule?
In
organic chemistry, carbyne is often a 'general term' for any
compound whose molecular structure includes an electrically
neutral carbon atom with three non-bonded electrons, connected
to another atom by a single bond. The chain of carbon atoms
is held together by either double or alternating single and
triple atomic bonds. That makes it a true one-dimensional
material, unlike atom-thin sheets of graphene.
Carbon chains are
traditionally classified as cumulene (monatomic chains with
double bonds, = C = C = ) or polyyne (dimerized chains with
alternating single and triple bonds, −C≡C−).
A polyyne
is any organic compounds with alternating single and triple
bonds; that is, (−C≡C−) n with n greater than 1. The
simplest example is diacetylene or buta-1,3-diyne, H−C≡C−C≡C−H.
Linear acetylenic carbon (also referred to as carbyne) is
an allotrope of carbon that has the chemical structure (−C≡C−)
n , with alternating single and triple bonds would
thus be the ultimate member of the polyyne family.
A
cumulene is a hydrocarbon with three or more
cumulative (consecutive) double bonds. A member of this compound
class is butatriene (which is also called simply cumulene),
H2C=C=C=CH2. Unlike most alkanes and alkenes, cumulenes tend
to be rigid, which makes them appealing for molecular nanotechnology.
Cumulenes are found in regions of space where hydrogen is
rare.
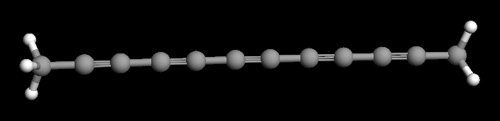
|
JPEG image of 12 carbon
chain carbyne molecule containing alternating carbon
single and triple bonds. The image was created using
the free software package ArgusLab.
|
|
|
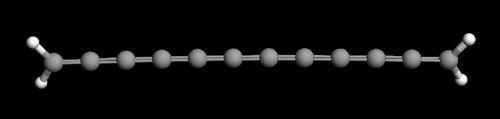
|
JPEG image of 12 carbon
chain carbyne molecule containing all carbon double
bonds. The image was created using the free software
package ArgusLab.
|
|
|
3 D STRUCTURES USING JMOL
Note:
All molecules on this page were built using Argus Lab 4.01
(see
free molecular modeling software). The structures were
Geometry Optimized in Argus Lab using the semiempirical method
(AM1 Hamiltonian).
BOND LENGTHS IN CARBON CHAINS
The
bond lengths of carbon chains characterizes the type of bond
between the carbons, For example
alkanes have a bond length of 1.51 ˚A , alkenes
1.33 ˚A and alkynes
1.19 ˚A. This however is not the case with carbynes (See below)
To
measure bond lengths with Jmol -- double click with left
mouse button twice on first atom, then drag cursor to second
atom and double click.
|
[Carbyne show with alternating carbon
single and triple bonds using the Jmol Applet] |
Try
this:
Rotate the Carbyne molecule to see the alternating single
and triple bonds
(Hold
the left mouse button down over the java applet image
and move the mouse to rotate the graphite molecule).
| | |
|
[Carbyne shown with alternating carbon
double bonds using the Jmol Applet] |
Try
this!!
Rotate
the Carbyne molecule to see the consecutive double bonds
|
|
|
Readings
and References
All-carbon
sp-sp2 hybrid structures: Geometrical properties, current
rectification, and current amplification --Scientific
Reports Sept. 2013
Gaining
knowledge of single carbon chains --Radboud University
Nijmegen Sept. 2011
The
[18] all-carbon molecule: cumulene or polyacetylene --J.
Am. Chem. Soc., 1991.
| 
