| Water
and Ice Side-by-Side
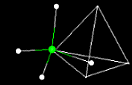
In
liquid water each molecule is hydrogen bonded to approximately
3.4 other water molecules. In ice each each molecule is hydrogen
bonded to 4 other molecules.
Compare
the two structures below. From observing these two structures
can you explain why ice is less dense than water?
| |
__ |
|
To
Rotate hold left mouse button down over molecule and
drag cursor.
Click the
right mouse button over the image to change from wireframe
to cpk, stick or ball and stick.
Return to --Water and
Ice PartI_
|