| Water
has important effects on all biological systems. What makes water so unique are
two very important properties. Water
is a polar molecule 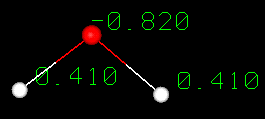 A water molecule is formed when two atoms of hydrogen bond covalently with an
atom of oxygen. In a covalent bond electrons are shared between atoms. In water
the sharing is not equal. The oxygen atom attracts the electrons more strongly
than the hydrogen.This gives water an asymmetrical distribution of charge. Molecules
that have ends with partial negative and positive charges are known as polar molecules.
It is this polar property that allows water to separate polar solute molecules
and explains why water can dissolve so many substances.
A water molecule is formed when two atoms of hydrogen bond covalently with an
atom of oxygen. In a covalent bond electrons are shared between atoms. In water
the sharing is not equal. The oxygen atom attracts the electrons more strongly
than the hydrogen.This gives water an asymmetrical distribution of charge. Molecules
that have ends with partial negative and positive charges are known as polar molecules.
It is this polar property that allows water to separate polar solute molecules
and explains why water can dissolve so many substances.
Water
is highly cohesive . 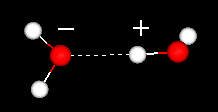 The positive regions in one water will attract the negatively charged regions
in other waters. The dashes show the hydrogen bond. In a hydrogen bond a hydrogen
atom is shared by two other atoms. The donor is the atom to which the hydrogen
is more tightly linked. The acceptor (having a partial negative charge) is the
atom which attracts the hydrogen atom. Click here
or on the image to your left to view a movie of two water molecules.
The positive regions in one water will attract the negatively charged regions
in other waters. The dashes show the hydrogen bond. In a hydrogen bond a hydrogen
atom is shared by two other atoms. The donor is the atom to which the hydrogen
is more tightly linked. The acceptor (having a partial negative charge) is the
atom which attracts the hydrogen atom. Click here
or on the image to your left to view a movie of two water molecules.
Liquid
water has a partially ordered structure in which hydrogen bonds are constantly
being formed and breaking up. 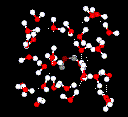 See a flash movie of water molecules in action.
See a flash movie of water molecules in action.
Hydrogen bonds
are much weaker than covalent bonds. However, when a large number of hydrogen
bonds act in unison they will make a strong contributory effect. This is the case
in water. 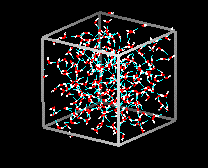 View Flash animation of this image.
View Flash animation of this image.
On
the other hand ice has a rigid lattice structure. 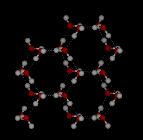
In liquid water
each molecule is hydrogen bonded to approximately 3.4 other water molecules. In
ice each each molecule is hydrogen bonded to 4 other molecules. Compare
the two structures below. Notice the empty spaces within the ice structure.
In
ice Ih, each water forms four hydrogen bonds with O---O distances of 2.76 Angstroms
to the nearest oxygen neighbor. The O-O-O angles are 109 degrees, typical of a
tetrahedrally coordinated lattice structure. The density of
ice Ih is 0.931 gm/cubic cm. This compares with a density of 1.00 gm/cubic cm.
for water. There
are eleven different forms of crystalline ice that are know. The hexaganol form
known as ice Ih is the only one that is found naturally. The lattice structure
of ice 1h is shown here.  Image Image
|